Hey internet,
Sorry I've been away for so long. I've had a crazy summer of conferencing and sciencing and research, so I reckon I should try and recap a bit of it for posterity and (hopefully) your enjoyment. In order to avoid getting any closer to what is already near tl;dr territory, I've split up the megapost into three slightly lesser posts.
First up: the European Materials Research Symposium!
I was lucky enough this June to present at the eMRS in Strasbourg, France. If you're interested in materials research and are looking for a big international conference, I would definitely recommend checking into this one. The fact that it was in France didn't hurt, either. The conference was a lot larger than I had expected-- about 2000 people by my estimation, all of which I met while in line to use the public internet kiosks. The city of Strasbourg was in all ways fantastic; lots and lots of culture and history without feeling too large or touristy.
The presentations were in general excellent, though biased slightly towards the physics and engineering side of materials. I can't really complain too much about chemistry getting the cold shoulder, because the big draw for the plenary lectures was Dr. Alan J. Heeger! Yes, that Dr. Alan J. Heeger![1]
Dr. Heeger gave a presentation on organic photovoltaics based on a paper that just came out in Nature Photonics (doi: 10.1038/nphoton.2009.69).
One of the grandfathers of the conjugated polymer field, Dr. Heeger also happens to be an excellent public speaker. It's awesome to see such spectacular research presented such that the general audience can understand it and even get excited about it (and, at a fairly technical conference too!). Dr. Heeger even went as far as to bring out a prototype organic solar cell that was generating about 2W just from the stage lights (of course, it helps when you have your own company that makes PVs).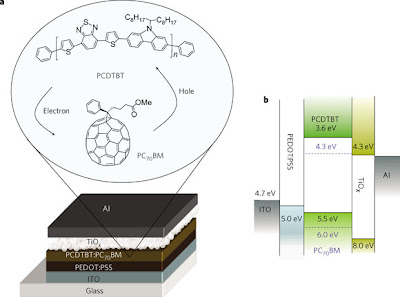
From what I could gather from his talk, organic solar cells have three tasks they need to tackle to get energy out of light:
- Efficiently absorb the light.
- Separate the excited state into charges, but real quick like (50 fs or less).
- Collect the charges.
- Profit!
To figure out all three of those things at once is tricky, to say the least (otherwise, we'd be looking at efficiencies above the record 5%-- still a long way away from any commercial possibility in my opinion). Heeger is working on systems known as bulk heterojunctions (BHJs), which are an interpolating network of donor/acceptor materials (in this work, conjugated materials as the hole transporter/donor and a modified fullerene as the electron transporter/acceptor), with domain features on the order of the nanoscale (to be more precise, close to half of the mean free path of the exciton, generally 10 nm or less).
What the little tiles in the above figure are supposed to show is that in a properly designed cell, there is a "waterfall" of energy levels leading down in the LUMOs (so the electron naturally falls "down" to the Al cathode) and leading up in the HOMOs (so the hole trickles "up" to the ITO anode).
The main issues with the current polymer-based BHJs are:
- The de-facto standard for the conjugated polymer, poly-3-hexylthiophene (P3HT), has a bandgap that is too large (about 1.9 eV, around 650 nm), which prevents a significant portion of the solar spectrum from being absorbed. An ideal material would have a bandgap close to about 1.2 eV to match the output from Mr. Friendly Sun.
- The energy difference between the HOMO of P3HT and the LUMO of the fullerene acceptor is too small, which limits the open-circuit voltage of these cells, which majorly contributes to the overall charge generating efficiency.[2]
The bandgap, however, remains fairly large-- 1.88 eV. Dr. Heeger noted that the efficiency of the cell under monochromatic green light (532 nm) is as high as 17%, which is almost as good as a super expensive crystalline Si cell! Unfortunately, the real world does not operate with monochromatic green light (or maybe fortunately, I don't think I'd like living in Oz too much), and the quantum efficiency of these cells is nearly 100%! That implies that the science is fundamentally solid, but the energy levels need to be engineered to capture more of the solar spectrum and increase the efficiency.

I should also give a shout out to Professor Efrat Lifshitz, at the Israel Institute of Technology. Dr. Lifshitz presented work on single dot spectroscopy in multiple exciton generation (MEG) in type-II core-shell quantum dots. MEG is a hot topic in the field of quantum dots for photovoltaics, catching the eye of Dr. Paul Alivisatos (a character that will make an appearance in the next chapter of my summer recap), among many others. The research involved definitely deserves a post of its own at some point in the future, but is extremely full awesome. Which says a lot when you're talking about extremely involved spectroscopy. Here's the paper she presented on, if you're into that sort of thing. Which I am.
Next up: a hopefully less wordy post on my adventures at the Advanced Light Source at Argonne National Laboratory in Illinois!
[1] Note to the conference organizer who introduced Dr. Heeger: the next time you invite a Nobel Laureate to speak at your conference, make sure you know his first name! I've no idea who Alex Heeger is, but apparently he does very nice things in conjugated polymers.
[2] Argh, the concept of Voc baffles and confounds me. Anyone care to clarify this (say, for instance, a certain expert in the field)? I know you want a large open circuit voltage, which somehow relates to the energy level gap, but as to why I cannot explain.

4 comments:
me? expert? you're very funny :)
i tried to explain Voc and related concepts here...is further clarification needed? i've been working on these things for so long that the basics are almost second nature, and it's easy to forget how little exposure to these concepts most people get...
Hi Jes! Thanks for commenting!
I re-read your post, and it makes sense on how to derive the Voc and Jsc from those C-V curves. I just don't have an intuitive understanding of what those values actually mean as a chemist who has successfully avoided electrochemistry like the plague up until this point. Why is a large donor HOMO and acceptor LUMO gap desireable for a large Voc?
Ahh. C-V curves make brain hurt. Ouch.
i like to avoid CV too--IMO, energy levels are better determined by UPS. ;)
Upon photoexcitation, an electron is excited from the donor's HOMO to the donor's LUMO, leaving a hole in the donor's HOMO. This bound electron-hole pair is called an exciton, and if you're lucky and it's able to reach the donor-acceptor interface, the electron can be transferred from the donor's LUMO to the acceptor's LUMO. (You need the acceptor's LUMO to be at least ~0.3-0.4 eV lower than the donor's LUMO for that to happen, and a similar offset for the HOMO levels so that holes can be transferred from acceptor HOMO to donor HOMO--because the acceptor is probably absorbing light too.) The simplified picture that a lot of people present is that the energy difference between donor HOMO and acceptor LUMO is the Voc--and the larger the Voc, the higher the power conversion efficiency that's possible, so you definitely want a large dHOMO-aLUMO gap.
(Of course, the Voc depends on a lot of other factors that aren't easily determined, so it actually ends up a little smaller than the aforementioned dHOMO-aLUMO gap...but that's going into way more detail than most people care about.)
Thanks Jes! That definitely helps. And I am definitely going to check this Igor thing out.
Post a Comment